Shaping Solutions for Remote Care and Communications
Investigating the origins of disease outbreaks and transmissions is key to advising urgent responsive actions in affected areas. To dramatically improve the efficiency of this critical task, APL researchers are applying an advanced tool called ChainChecker to help responders visualize data and make quick, decisive actions in the field. The APL-enhanced system combines epidemiological and genomic data to track disease transmission. Initially developed by the Centers for Disease Control and Prevention (CDC) and Imperial College London, ChainChecker, bolstered by four years of APL development, has become pivotal to determining outbreak responses.
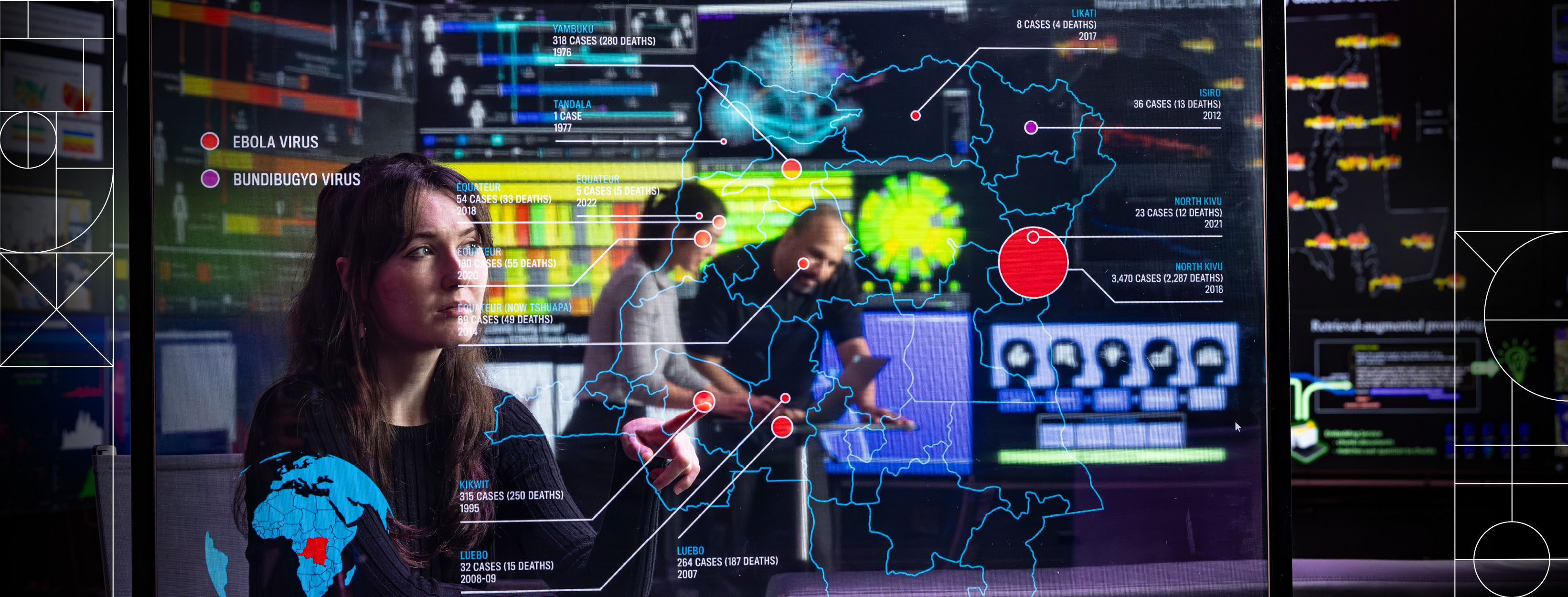
In work highlighted in the journal The Lancet Microbe in February 2024, the APL team applied the tool to investigating the 2020 Ebola outbreak in the Democratic Republic of the Congo retrospectively to prove the ChainChecker’s power. The outbreak involved concurrent clusters of the virus on opposite sides of the country, a distance that complicated tracing the chains of transmission and muddied any potential connection between the clusters themselves. ChainChecker helped researchers visualize how the virus spread and understand the outbreak’s dynamics; scientists confirmed two Ebola subtypes, one likely originating from a survivor of a previous outbreak. This dual analysis of genetics and transmission patterns underscored the utility of the approach for understanding disease dynamics.

Julia Eng and Miles Stewart monitor outbreak status in APL’s Live Data, Integration, Validation and Experimentation (LIVE) Lab. APL’s advanced tools are improving the ability of health care responders to rapidly and accurately interpret data and make better decisions in the field during a disease outbreak.
“ChainChecker is giving responders quick and critical information on novel transmission modes, infection prevention and control measures, and other aspects of an outbreak,” explained Miles Stewart, an APL project lead and software engineer. “Our advanced tools are improving the ability of responders to make better decisions in the field during an outbreak through rapid, more accurate interpretation of data.”
Beyond Ebola, ChainChecker is being applied to other viral hemorrhagic fevers, like Marburg virus disease. APL’s collaboration with the CDC and international health agencies is improving response strategies and ensuring faster, more accurate interpretations of outbreak data.
“From data analysis to interdisciplinary collaboration, APL’s capabilities are changing our approach to complex biological challenges,” Stewart said.
Among those challenges is giving medics an easier way to deliver lifesaving care to people injured in remote conflict zones or natural disasters. APL researchers leveraged the power of augmented reality, predictive anatomy visualization and artificial intelligence (AI) to develop a tool that makes it possible to “see” beneath the skin and predict where organs are situated, said Anna Knight, an APL biomedical engineer who leads medical image integration for the project.

Using APL’s AI-boosted anatomy visualization aid, a medic can spot the probable location of an internal injury through visible landmarks on a patient’s body.
The anatomy visualization aid relies on a statistical shape atlas — a detailed map of variations in human anatomy that the team built with hundreds of CT scans chosen to match the gender, ethnicity and body shape diversity of warfighters in the U.S. military. A user can predict the probable location of an injury in specific organs through visible landmarks on the patient’s body. Then, with an augmented reality headset, medics can see a digital overlay of the patient’s predicted anatomy.
“This tool could help to address a major challenge in field medicine, making it so medics can envision what lies beneath the surface and guide emergency response,” said Bobby Armiger, the project’s principal investigator and head of exploratory science for APL’s Research and Exploratory Development Department. Preliminary results showed the models can predict the individual shape of 66 different anatomical structures within the thorax alone.
The entire project sprang from medics’ need to improve trauma care on the battlefield. Recent shifts in American military engagements, characterized by prolonged conflicts in remote regions (such as Afghanistan) necessitate prolonged care in the field, underscoring the need for advanced trauma care in austere environments. Where in the past airlifting individuals to safety was a key part of the strategy, Armiger said the goal is to enable more self-sufficient, immediate care on the battlefield and in remote areas. The effort fits squarely within the APL Global Health Mission Area’s focus on assured care, counteracting natural, deliberate and accidental health threats through groundbreaking science and engineering.
“We’re developing technology that could one day contribute toward making care accessible wherever it’s needed the most,” said Alan Ravitz, chief engineer of the mission area.
While augmented reality is still uncommon on the battlefield, applications like this are geared toward enhancing medic training in the near term and may one day enable any service member to deliver battlefield medicine.
APL researchers are also refining methods to predict how someone will react to a pathogen, disease or treatment. The Immunity Twin project — initially funded as part of APL’s Innovation Program as a Propulsion Grant — combines biological data with advanced computational tools; and with touches of machine learning and systems modeling, an APL team is building a dynamic model of how the human immune system might react to various conditions.
“Our objective is to develop both digital and in vitro models of a person’s immune system to predict responses to diseases or treatments,” said Sarah Grady, a molecular biologist and the project’s principal investigator. “We are targeting the unpredictability in immune system responses, aiming for a future where personalized medical interventions are commonplace.”
To address this challenge, APL pulled on the multidisciplinary collaboration few institutions can match, assembling a team of virologists, computational modelers and data scientists to create both physical and digital replicas of immune systems. They’re focused on the respiratory system, studying how different lungs respond to the flu — a common virus that can be especially harmful to vulnerable populations.
Our objective is to develop both digital and in vitro models of a person’s immune system to predict responses to diseases or treatments. We are … aiming for a future where personalized medical interventions are commonplace.
— Sarah Grady, molecular biologist and principal investigator for Immunity Twin
According to APL biologist and project co-investigator Molly Gallagher, the integration of lab work and computational modeling ensures the team is collecting data that directly informs the digital model — separating the APL effort from similar work at other institutions.
Another unique aspect of APL’s approach is the use of “cell painting,” in which, by staining cells with fluorescent dyes, researchers can image various cell structures under a microscope. The difference? In a first, APL has modified the cell-painting method to work with living lung organoid cells, allowing the observation of real-time responses to viral infections.
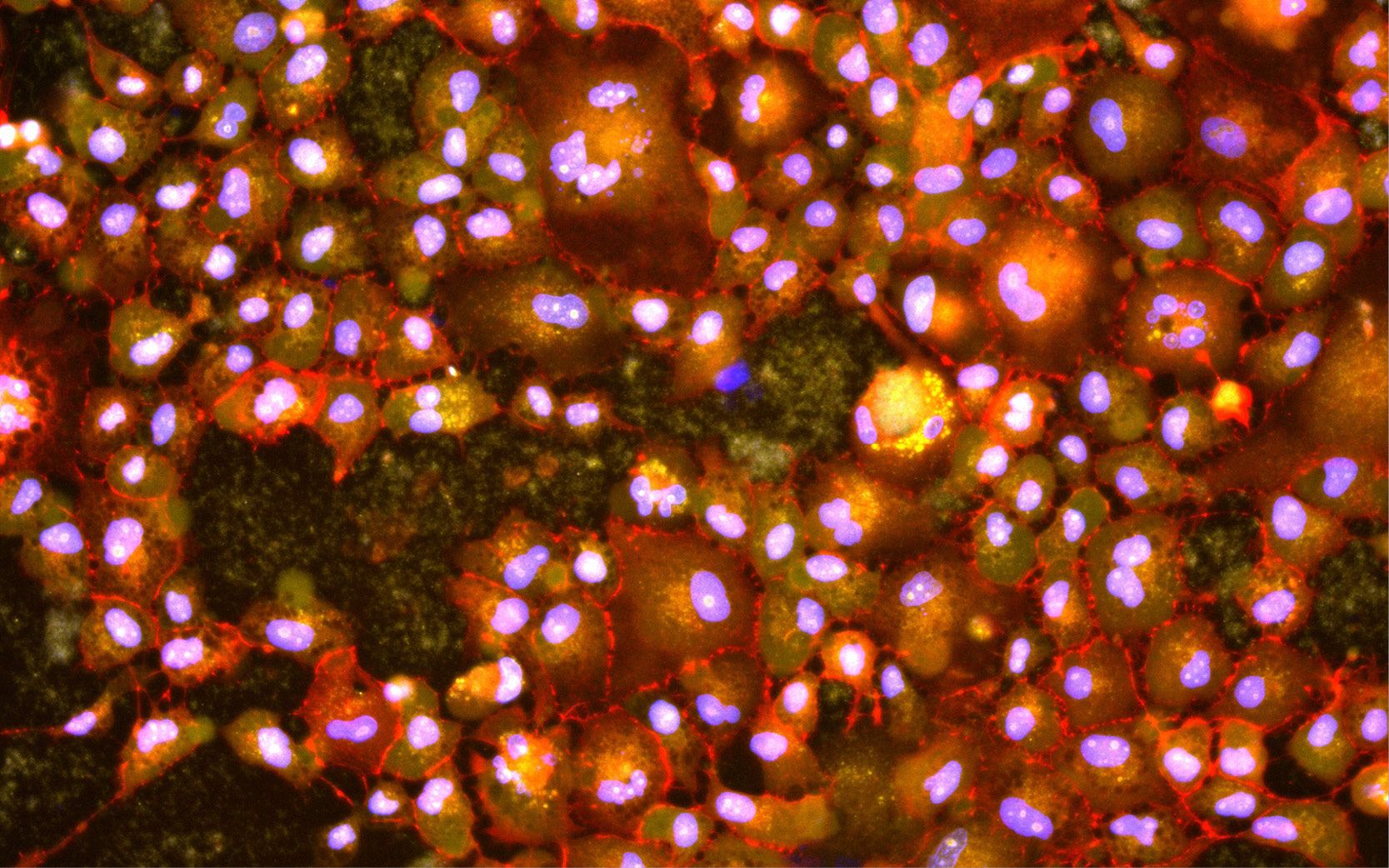
By staining cells with fluorescent dyes, researchers can image cell structures under a microscope. APL is the first to use “cell painting” on living cells, allowing the observation of real-time responses to viral infections.
“This allows us to observe real-time responses to viral infections,” Gallagher explained. “We can see what’s happening inside the cells over time, giving us more accurate data.”
APL also uses advanced 3D tissue culture models that mimic human lungs, providing more sophisticated insights than traditional single-cell models. Coupled with cutting-edge imaging systems, this approach enables researchers to collect real-time data and account for genetic differences that affect immune responses.
A key element of the project is its iterative approach to data integration. The team uses biological data to build its digital models, which are continuously refined through further experiments. This feedback loop is critical for developing predictive models with real-world applications that extend beyond individual health care to include military and pandemic preparedness.
“Our models are built to predict how the immune system might respond to new challenges,” said Grady, referencing the team’s work related to influenza. “With accurate simulations, we can make more personalized decisions about treatments and vaccines.”
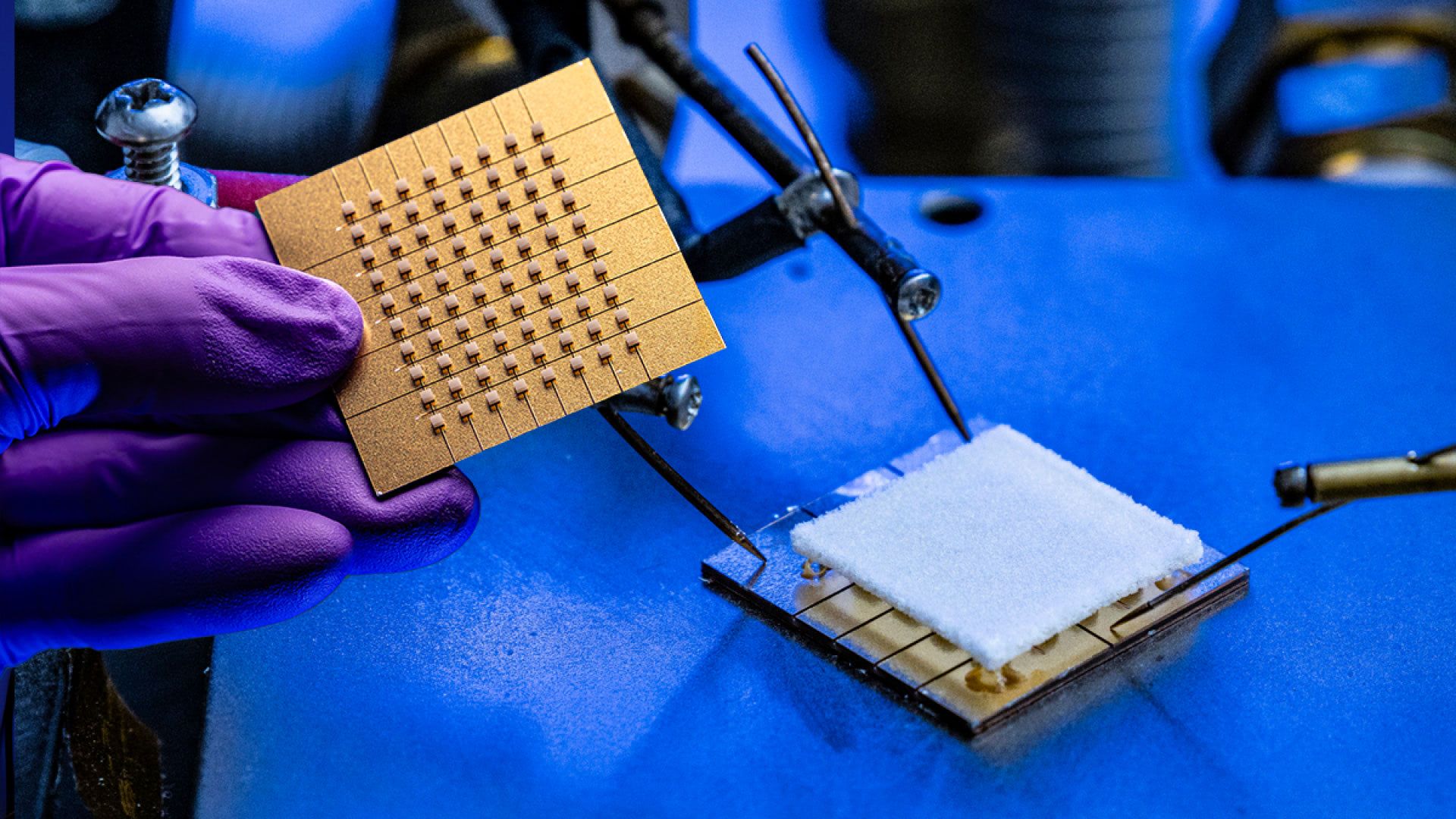
APL is also contributing to the development of a prototype miniaturized cellular system that may one day keep warfighters and first responders connected in remote environments. Through the Standalone On-the-Move Advanced Relay (SOAR) program, APL aims to create a 5G system that can strap to a small drone or tethered balloon and provide local cell service to a wide area in a tactical environment. The entire package — antenna, mechanical box and heat-dissipation systems — is just under 10 pounds (0.45 kilograms) and smaller than a shoebox.
Such systems allow the military to control various devices with cheaper radios, says Sean Brassard, an engineering program manager in APL’s Department of Defense Special Operations Program Area. SOAR can be used for tactical military missions and disaster relief when communications are down — all supported by APL expertise in cellular engineering and design, from individual devices like smartphones to the enterprises supporting them.
The team demonstrated the prototype SOAR 5G system in April and field tested it in June, using drones tethered to power cables that provided hours of operation. Next up: starting a second version of the system that will operate on free-flying drones.

(From left) Kevin Honsaker, Tamoor Hamid and Jon Platt prepare a SOAR system for flight. The box attached to the drone’s underside contains a 5G wireless module, and an APL-designed antenna is below the module. Credit: U.S. Army/Dan Lafontaine